
New potential target to kill cancer cells identified by USask researchers
A team of University of Saskatchewan (USask) researchers believe that iron metabolism could be targeted to treat an aggressive form of cancer.
By Matt Olson, Research Profile and Impact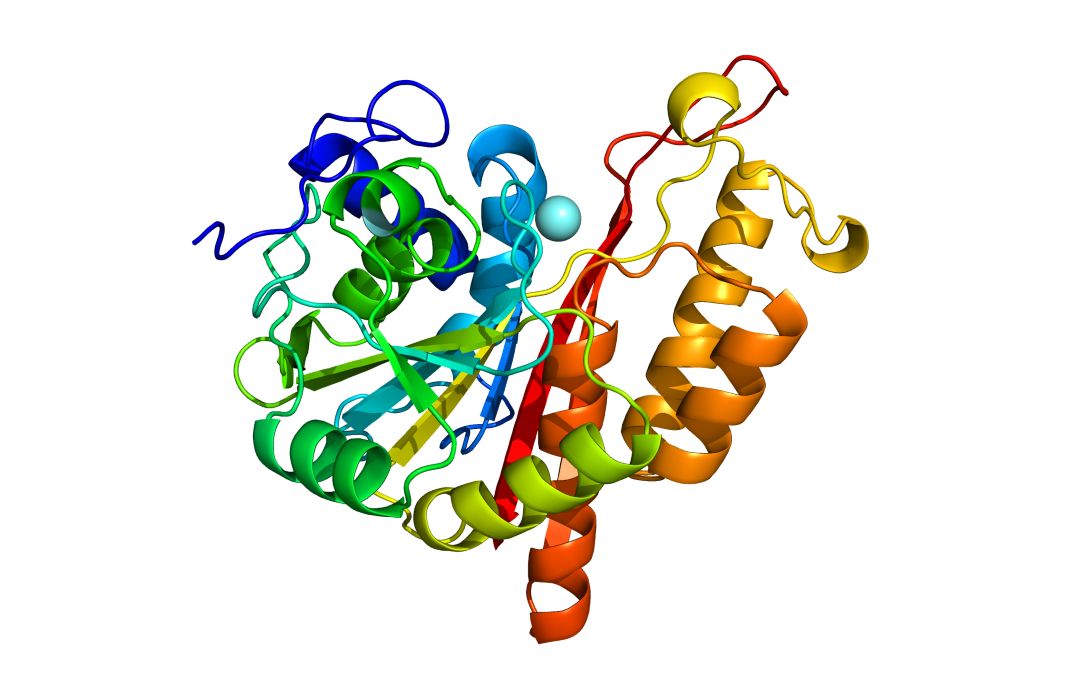
Dr. Oleg Dmitriev (PhD), a professor in the department of Biochemistry, Microbiology and Immunology, and oncology professor Dr. Franco Vizeacoumar (PhD) in USask’s College of Medicine were two of the authors of a research paper recently published in eLife identifying the function of a less-understood cell protein referred to as MEMO1.
As Dmitriev puts it, the team has discovered that MEMO1 binds iron and regulates iron flow in the cell. And because MEMO1 appears at high levels in triple negative breast cancer (TNBC) cells, Dmitriev said this could make iron regulation – and the MEMO1 protein – a potential target for cancer treatments.
“The fundamental interest for us (in the protein) is the regulation of metals in the cell, and then the medical importance is its role in cancer,” he said. “It looks like MEMO1 makes cancer cells more sensitive to disruptions of iron distribution, so the practical upshot is that we can use iron metabolism in the cell as a target to kill cancer cells.”
Researchers are still investigating the primary function of the MEMO1 protein in cells, but Dmitriev, Vizeacoumar and their colleagues have determined that it plays an important role in the regulation of iron metabolism in the cell – or in other words, the traffic of iron into and out of the cell and how it ends up being used.
MEMO1 is one of many proteins involved in balancing iron in the cell. But through detailed genomic analysis, Dmitriev said they can identify which proteins work in conjunction with MEMO1, and tracing the network of protein interactions can lead to the core function of MEMO1 itself.
Researchers could then create drugs that target MEMO1 or its interacting protein partners and kill cells producing high levels of MEMO1 – like TNBC – by disrupting their iron regulation.
TNBC is one of the most aggressive and difficult-to-treat types of cancer, so a new possible target for treatment that lacks the side effects of standard chemotherapy could have major benefits for cancer patients.
“The problem is many cancer drugs are very powerful but also terribly toxic,” Dmitriev said. “If you can identify a drug that is effective specifically in the cells producing large amounts of MEMO1, chances are you can use that drug at a much lower dosage to treat high-MEMO1 cancers.”
Dmitriev lauded Vizeacoumar and their collaborators on the project, noting the possibilities of combining knowledge from different areas of science to solve complex biomedical problems.
He said that it’s important for research scientists to both see and show how their work will affect people’s lives, and this research is the very beginning of new medical treatments that have tremendous potential.
“It's extremely important for us scientists not to get isolated in our ivory tower,” Dmitriev said. “There is nothing like talking to cancer patients and those who beat the disease to realize the need for new treatments.”
Together, we will undertake the research the world needs. We invite you to join by supporting critical research at USask.

